Courtesy of Dr. Agustín Vecchia.
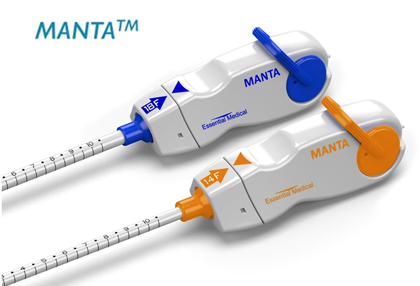
The following prospective, multicentre study was conducted in three European institutions and was aimed at assessing the safety and efficacy of the MANTA vascular closure device system, designed for 10- to 25-Fr-arteriotomy closure. This device contains a closure unit consisting of polylactic acid (intraarterial toggle), a bovine collagen (extravascular) pad, a nonresorbable polyester suture connecting both, and a stainless steel (nonresorbable) suture lock.
The study enrolled 50 patients with a mean age of 79.5 years, who underwent the following:
- complex coronary angioplasty,
- ballon aortic valvuloplasty, or
- transcatheter aortic valve replacement with large-bore catheters (12 to 19 French, i.e., 4 to 6.33 mm).
MANTA closure was performed by 9 different operators. Almost the whole group (47 out of 50 patients) underwent TAVR. The mean time to hemostasis with the MANTA device was 2 minutes, 23 seconds.
One patient experienced a major vascular complication that required a covered stent and subsequent surgical correction (this patient required emergency surgery for an incorrectly-placed TAVR device). One patient experienced minor bleeding (VARC-2) and one patient experienced a pseudoaneurism. Mortality at 30 days was 8% (n = 4); there were no device-related mortality events. Five patients presented subcutaneous bruising that did not require treatment. The control angiography conducted after the procedure showed patency in the femoral artery in all patients.
Conclusion
The authors conclude that the MANTA device demonstrates safety and rapid hemostasis for large-bore arteriotomy closure.
Editorial
The importance of this study lies in the fact that most vascular complications observed in large-bore arteriotomies (regardless of the procedure) derive from closure flaws, and MANTA offers the first alternative in that sense.
Something to be highlighted is the fact that, although none of the 9 operators in this study had previous experience with the device, this was placed correctly in all patients (in a clinical study context), with acceptable rates of both minor and major complications. This is particularly surprising, taking into account the complication rates observed in recent studies such as “CONTROL” (Closure Device in Transfemoral Aortic Valve Implantation), which enrolled 944 patients undergoing TAVR and compared Prostar XL vs. Perclose Proglide (Abbott Vascular, Redwood City, California). In that case, the rate of major and minor bleeding, according to the VARC classification, was 7.4% and 14.8%, respectively, for patients in the Prostar arm, and 1.9% and 18%, respectively, for patients in the Proglide arm.
Courtesy of Dr. Agustín Vecchia. Buenos Aires German Hospital, Argentina.
Original title: Percutaneous Plug-Based Arteriotomy Closure Device for Large-Bore Access A Multicenter Prospective Study.
Reference: DOI: 10.1016/j.jcin.2016.12.028 JACC Cardiovascular Interventions.
Authors: Nicolas M. Van Mieghem, MD, PHD, Azeem Latib, MD, Jan van der Heyden, MD, PHD, Lennart van Gils, MD, Joost Daemen, MD, PHD, Todd Sorzano, Jurgen Ligthart, RT, Karin Witberg, CCRN, Thom de Kroon, MD, Nathaniel Maor, Antonio Mangieri, MD, Matteo Montorfano, MD, Peter P. de Jaegere, MD, PHD, Antonio Colombo, MD, Gary Roubin, MD, PHD.
Subscribe to our weekly newsletter
Get the latest scientific articles on interventional cardiology
We are interested in your opinion. Please, leave your comments, thoughts, questions, etc., below. They will be most welcome.