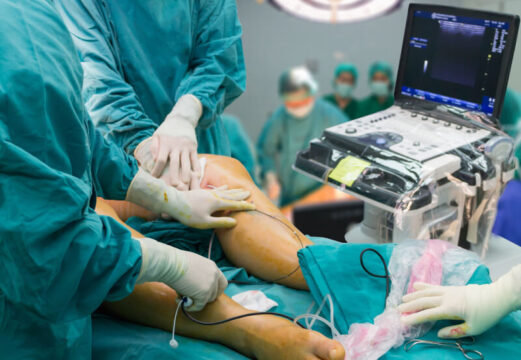
This study aimed to assess the outcome of Culotte stenting with newer-generation drug-eluting stents (DES) in Medina 1, 1, 1 bifurcation lesions. The 2nd-generation device used was permanent-polymer everolimus-eluting stent Xience, a device for which there is comparatively plenty of evidence available. Alternatively, the 3rd-generation stent used was thin-strut abluminal bioresorbable-polymer everolimus-eluting stent SYNERGY.
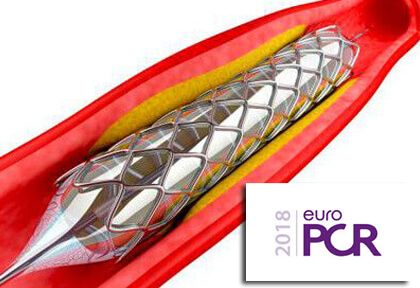
The rate of combined events (death, acute myocardial infarction, stroke, target-vessel failure, stent thrombosis, or binary restenosis) occurred in 19% of the Xience group vs. 16% of patients in the Synergy group, which means that the latter reached the non-inferiority criterion.
Original title: Culotte Stenting for Coronary Bifurcation Lesions with 2nd and 3rd-Generation Everolimus-Eluting Stents: The CELTIC Bifurcation Study.
Presenter: David P. Foley.
Subscribe to our weekly newsletter
Get the latest scientific articles on interventional cardiology
We are interested in your opinion. Please, leave your comments, thoughts, questions, etc., below. They will be most welcome.