The US Food and Drug Administration (FDA) has cleared the SAPIEN 3 valve with the Alterra adaptive pre-stent for implantation in pulmonary position.
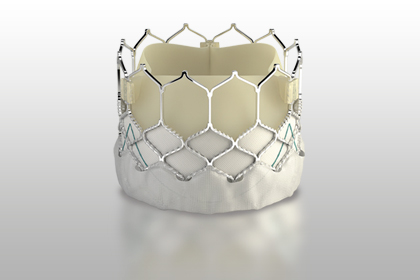
According to Edwards Lifesciences, this new system developed for pulmonary valve is capable of compensating a wide variety of sizes and morphologies of the right ventricular outflow tract, allowing for stable valve implantation.
Patients with congenital heart disease who have already undergone multiple corrective cardiac surgeries during their lifetime are pleased with the approval of this new tool.
For these patients, Alterra is one more opportunity with a much less invasive procedure.
The FDA approved the device for the management of children or adults with severe pulmonary insufficiency shown in an echocardiogram who have a native or surgically repaired outflow tract and an indication for pulmonary valve replacement.
Earlier this year, the same regulatory body approved the Harmony device, a catheter-based pulmonary valve produced by Medtronic.
Harmony was the first nonsurgical valve approved to treat pulmonary insufficiency in pediatric or adult patients with native or repaired outflow tracts.
The catheter-based valve competition is booming (for all four valves), and there are still many devices with potential advantages going through the approval process.
Read also: Women Present Lower Risk of Sport Related Sudden Death Compared to Men.
We will undoubtedly have many similar news in the next months that will widen our range of possibilities for better treatments.
Original title: Edwards LifeSciences. Edwards receives FDA approval for SAPIEN 3 with Alterra prestent for transcatheter pulmonic valve replacement.
Reference: Publicado por la FDA el 20 de diciembre 2021.
Subscribe to our weekly newsletter
Get the latest scientific articles on interventional cardiology





