For certain structural interventions and procedures in the field of interventional electrophysiology, complex venous access (CVA) using large-bore introducers is required. While these large-bore venous accesses generally have a lower rate of adverse effects compared to arterial accesses, there still are associated complications. The use of percutaneous closure devices has proven effective in reducing the time to achieve adequate hemostasis and facilitating early ambulation, which could contribute to shorter hospital stays.
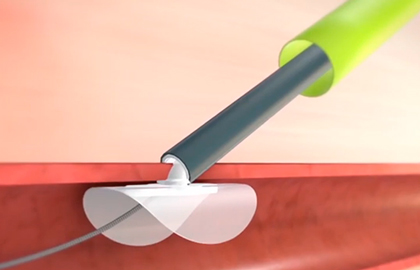
The Perclose ProGlide (PPG) device is a percutaneous closure system approved for both arterial and venous access. Its use in veins has primarily been assessed in pulmonary vein isolation procedures for the treatment of atrial fibrillation.
The purpose of the SAFE-VEIN trial, conducted by Ali et al. from the Aurora St. Luke’s Medical team in Milwaukee, was to evaluate the efficacy and safety of PPG compared to the “figure-of-eight” suture for venous access closure, assessing hemostasis in patients requiring a CVA.
The study enrolled patients undergoing electrophysiological and structural interventions, excluding those with skin or systemic infections, coagulation disorders, hypercoagulable states, or morbid obesity. Subjects were randomized 1:1. While the original study design included three arms (PPG vs. “figure-of-eight” suture vs. manual compression), manual compression was prematurely abandoned due to the need for transfusion due to bleeding.
Read also: Colchicine in Coronary Plaque Stabilization: Results According to OCT.
The primary efficacy endpoints were time to hemostasis (TTH) and time to ambulation (TTA). Secondary endpoints included time to hospital discharge (TTD), major or life-threatening bleeding, access-site hematoma, vascular thrombosis, pseudoaneurysm, or arteriovenous fistulae.
The procedure was performed using an ultrasound-guided micropuncture needle to ensure safe access. Two PPG devices were used for venous accesses > 13 Fr, and a single PPG for accesses <13 Fr. After access was obtained, researchers administered a 100-mg/kg heparin bolus followed by an infusion to maintain an activated clotting time (ACT) between 300 and 350 seconds. PPG placement varied by operator, and it was done either before or after removing the CVA.
The analysis included data from 100 patients with a follow-up of at least 30 days. The average age was 69.6 ± 11.0 years, and 63% of subjects were men. There were no baseline differences in the use of antiplatelet or anticoagulant therapies, and the use of protamine before removing the venous introducer did not vary between groups. The most common procedure among the randomized patients was left atrial appendage closure (51%).
Read also: Atherosclerotic Renal Artery Stenosis: To Revascularize or Not.
Following initial post-procedural hemostasis, 22 patients required supplementary use of FemoStop to achieve complete hemostasis, which was more frequent in the “figure-of-eight” suture group (32.1%) compared with the PPG group (10.6%).
Primary efficacy endpoint TTH was significantly shorter for patients in the PPG group (median in minutes, Q1, Q3 [7 (2, 10) vs. 11 (10, 15), p <0.001]). Regarding TTA, researchers observed the same trend, with significantly shorter times for the PPG group [322 (246, 452) vs. 403 (353, 633), p = 0.005]. However, there were no significant differences in TTD [1257 (1081, 1544) vs. 1338 (1171, 1435), p = 0.650].
Complications were low in both groups and there were no significant differences related to the randomized strategy.
CONCLUSIONS
In this study, the use of closure devices improved access recovery times compared to the “figure-of-eight” suture. However, this advantage did not translate into reduced time to hospital discharge, as there were no differences in TTD. The cost-effectiveness of closure devices in venous accesses remains unclear.
Original Title: Suture closure AFtEr large bore vein access (SAFE‐VEIN): A randomized, prospective study of the efficacy and safety of venous closure device.
Reference: Ali M, Masood F, Erickson L, Adefisoye J, Kanani J, Walczak S, Ajam T, Kieu A, Premjee M, Jan MF, Allaqaband SQ, Bajwa T, Khitha J, Zilinski J, Jahangir A, Djelmami-Hani M, Sra J, Niazi I, Mortada ME. Suture closure AFtEr large bore vein access (SAFE-VEIN): A randomized, prospective study of the efficacy and safety of venous closure device. Catheter Cardiovasc Interv. 2024 Aug 1. doi: 10.1002/ccd.31173. Epub ahead of print. PMID: 39087741.
Subscribe to our weekly newsletter
Get the latest scientific articles on interventional cardiology





