After reports of some serious adverse events with first-generation Lotus, the device was pulled off the market—but it was not meant to be left in oblivion. Now, it is back, renewed and with the approval of the United States Food and Drug Administration (FDA), to compete directly with the two market leaders (Sapien and CoreValve), which were the only devices approved for the US market so far.
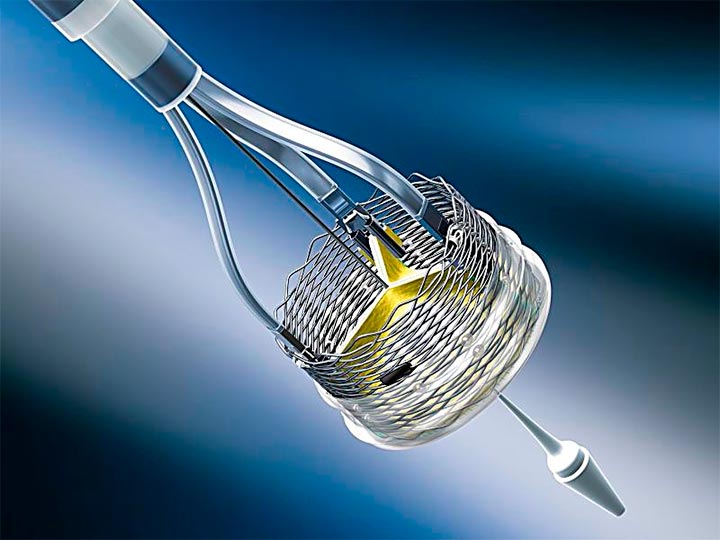 Starting yesterday, there are three percutaneous valves available in the US market; each embodies a different concept and presents advantages and disadvantages. This can be seen as a competition among these devices, but, actually, they will most likely end up supplementing each other. Our responsibility is to find the most appropriate valve for each patient.
Starting yesterday, there are three percutaneous valves available in the US market; each embodies a different concept and presents advantages and disadvantages. This can be seen as a competition among these devices, but, actually, they will most likely end up supplementing each other. Our responsibility is to find the most appropriate valve for each patient.
The FDA has just approved the transcatheter aortic valve replacement system LOTUS Edge for patients at high surgical risk.
Read also: REPRISE III: Lotus and CoreValve Compared in High-Risk or Inoperable Patients.
The FDA decision is supported by the results of the REPRISE III trial, which included the first-generation device and also patients who received the Lotus Edge valve.
REPRISE III outcomes were presented at EuroPCR 2017 and showed that the Lotus system was non-inferior to the results of a population receiving both first- and second-generation CoreValve (the latter, Evolut R).
Lotus criticism focused on its high rates of need for pacemaker, a problem somewhat solved through a modification to the original design, a change known as “depth guard.” This prevents excessively deep implantations and limits interaction with the outflow tract.
Read also: Lotus Valve in Real Life Patients: the near total lack of leaks is its greatest strength.
The REPRISE III 2-year follow-up (recently published) confirmed the first outcomes for the only valve in the market that allows for full delivery, function assessment, and (in case of unsatisfactory results, such as leak, block, etc.) full recapture, so as to optimize results as much as possible.
Patient enrollment for REPRISE IV, including Lotus Edge intermediate-risk patients, started early this year.
FDA approval might indirectly increase the use of cerebral protection devices during the procedure, since Boston bought the rights to the Sentinel device last year and there is much speculation about the valve and the cerebral protection device being released for sale as a combined package.
Original title: Boston Scientific Receives FDA Approval for LOTUS Edge Aortic Valve System. Published on: April 23, 2019. Accessed on: April 24, 2019.
Get the latest scientific articles on interventional cardiologySubscribe to our weekly newsletter
We are interested in your opinion. Please, leave your comments, thoughts, questions, etc., below. They will be most welcome.





