The Portico self-expanding graft has obtained FDA approval and can now compete as one of the three options available in clinical practice for transcatheter aortic valve replacement (TAVR) in the United States. For the time being, its indication will have some restrictions: for example, it can only be used in high-risk patients (unlike CoreValve and Sapien, both FDA-approved for the whole spectrum of patients).
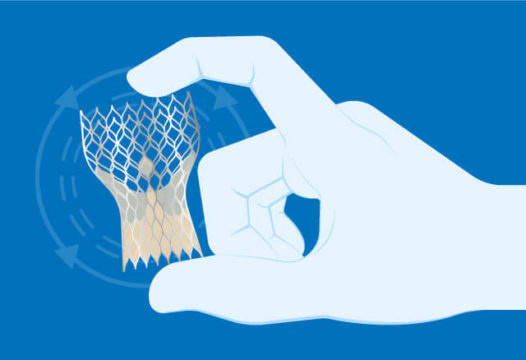
The results of the open, prospective trial were first presented during the TCT 2019 Congress, showing that the Portico valve reached non-inferiority against the other two devices approved for clinical practice.
At the time, the composite primary endpoint of safety (all-cause mortality, disabling stroke, life-threatening bleeding, acute renal failure requiring dialysis, or vascular complication >30 days) occurred in 13.8% of patients treated with the new device vs. 9.6% of patients treated with the traditional devices (pfor noninferiority = 0.03).
For the composite efficacy endpoint (death and disabling stroke at 1 year), the Portico valve reached 14.9% vs. 13.4%; it was also non-inferior (p = 0.006).
Additional data on the Portico were published during the London Valves that very same year. The Portico 1 study showed at 2 years an all-cause mortality rate of 19.7%, a cardiovascular death rate of 9.6%, and a stroke rate of 3.1%.
The pacemaker implantation rate was high—22.3% at 2 years—, although identical to that reported at 1 year.
Read also: TAVR and Anticoagulation: Direct Anticoagulant Agents or Vitamin K Inhibitors?
Moderate and severe leaks generated controversy and skepticism. At 2 years, their rate was 1.5%—far from the 7.8% reported at 1 year. These data clearly need more research in every sense of the word.
From now on, the Portico system joins the CoreValve/Evolut self-expanding family by Medtronic, and the Sapien balloon-expandable family manufactured by Edwards.
In total, three devices have been approved for use in clinical practice. What will be next?
Read also: The latest scientific articles on TAVI published on our website.
Boston Scientific had obtained FDA approval for the Lotus; however, after several issues, and because of its complexity, Boston dropped the device redesign and withdrew the valve from the market. Now all of Boston’s efforts are aimed at Acurate Neo2, a much simpler self-expanding system than the mechanically expandable Lotus. Will Acurate be the star of the next announcement?
Original Title: Abbott receives FDA approval for minimally invasive Portico with FlexNav TAVR system to treat patients with aortic valve disease.
Reference: Abbott, 20 de septiembre 2021.
Subscribe to our weekly newsletter
Get the latest scientific articles on interventional cardiology